Press Releases
* Please note that the news release contains the content at the time of the announcement and may differ from the latest information.
By continuous intake of the microalgae Euglena
Confirmed research results suggesting alleviation of influenza symptoms
Euglena Co., Ltd.
Euglena Co., Ltd. (Headquarters: Bunkyo-ku, Tokyo, President: Mitsuru Izumo) uses powder of microalga Euglena (Japanese name: Euglena) and continuous intake of paramylon * 1, which is a unique ingredient. We are pleased to inform you that we have confirmed the research results that suggest that the symptoms of influenza will be alleviated.
Influenza, which is prevalent every winter, is an acute respiratory tract infection caused by the influenza virus. This time, Euglena powder, paramylon and amorphous paramylon * 2 (hereinafter referred to as the test substance) were orally ingested by mice infected with influenza virus. As a result, it was confirmed that the mice that took the test substance had a significant improvement in survival rate and the number of influenza viruses in the lungs decreased compared to the mice that did not take the test substance.
The results of this research were presented at the 62nd Japanese Society of Virology on November 11, 2014. Going forward, we will continue to research the functionality of paramylon Euglena the medical field and increasing its added value as a food ingredient.
The details are as follows.
* 1 Paramylon: A polysaccharide in which glucose molecules are linearly polymerized by β-1,3-bonds.
* 2 Amorphous paramylon: Paramylon is chemically treated to destroy its crystal structure (non-crystalline).
Suggestions for alleviating influenza symptoms by continuous intake of the microalgae Euglena
■ Research content
(1) About the research to confirm the survival rate
Influenza virus (A / PR / 8/34 (H1N1)) was applied to mice that were allowed to freely ingest test substances (Euglena powder, paramylon, amorphous paramylon) and diet for 2 weeks, and mice that were allowed to freely ingest only diet (control group). It was administered nasally and the survival rate of each group was measured for 10 days.
As a result, it was confirmed that the mice ingested the test substance had a significant improvement in survival rate as compared with the mice in the control group.
(2) Confirmation research on the number of influenza viruses in the lungs
Influenza virus (A / PR / 8/34 (H1N1)) was applied to mice that were allowed to freely ingest test substances (Euglena powder, paramylon, amorphous paramylon) and diet for 2 weeks, and mice that were allowed to freely ingest only diet (control group). After nasal administration, the number of influenza viruses in the lungs was measured for 3 days.
As a result, it was confirmed that the number of influenza viruses in the lungs was significantly reduced in the mice ingesting the test substance as compared with the mice in the control group.
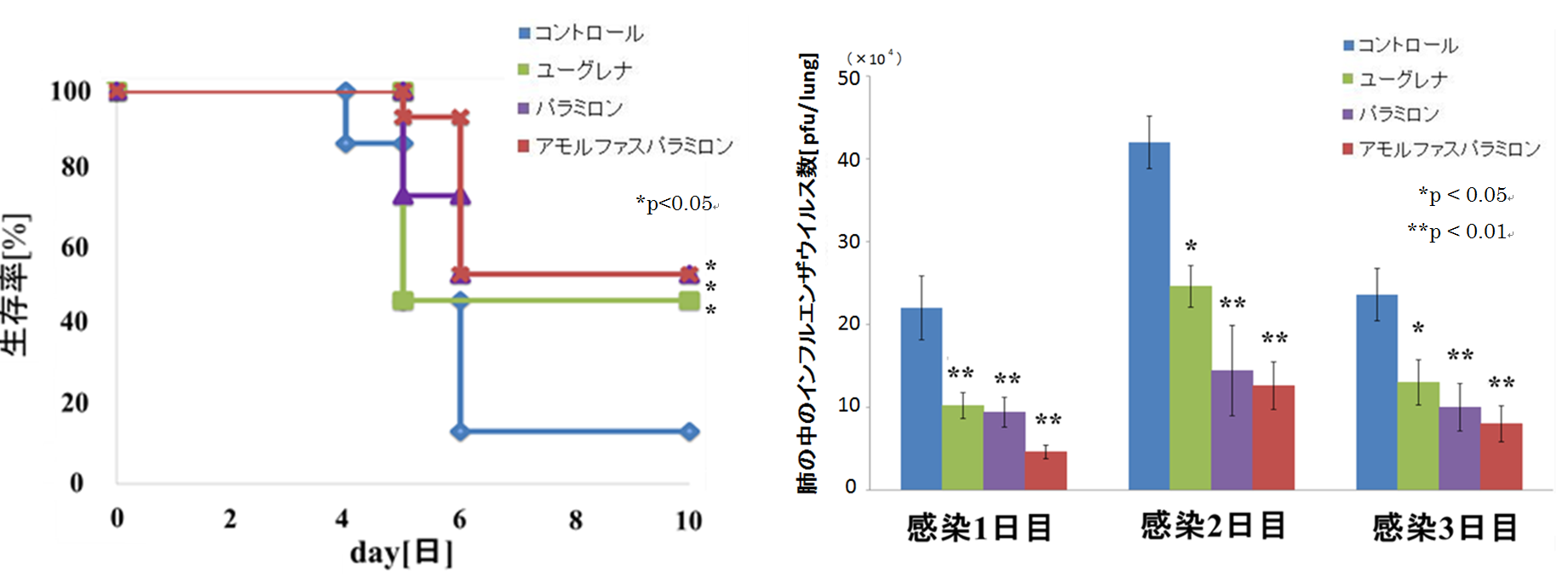
(1) Left figure: Changes in survival rate (2) Right figure: Changes in the number of influenza viruses in the lungs
■ Consideration
The results showed Euglena powder, paramylon, and amorphous paramylon may have a symptom-relieving effect on influenza virus infection.
-Contact for inquiries from the press-
Euglena Co., Ltd. Public Relations and IR Division
